【印刷可能】 ƒGƒ”ƒ@ƒ“ƒQƒŠƒIƒ“ •ÇŽ† Žg“k 426107
Cg gk questions in hindi pdf objective hindi general knowledge questions about chhattisgarh history, indian history in hindi quiz, computer gk, indian constitution gk, general hindi grammarQuestion 24 SURVEY 1 seconds Report an issue Q X (g) 2 Q (g) ⇄ R (g) Z (g) K c = 13 × 10 5 at 50°C A 10 mol sample of X (g) and a 10 mol sample of Q (g) are introduced into an evacuated, rigid 100 L container and allowed to reach equilibrium at 50°C according to the equation aboveAnswer (1 of 4) The numbers corresponding to the letter sequence are the prime numbers B, C, E, G, K, M, Q, S = 2, 3, 5, 7, 9, 13, 17, 19 The next prime in this sequence is 23 The number corresponding to the 23rd letter of the alphabet is W, the last letter of
French Greetings French Letters How Does The French
ƒGƒ"ƒ@ƒ"ƒQƒŠƒIƒ" •ÇŽ† Žg"k
ƒGƒ"ƒ@ƒ"ƒQƒŠƒIƒ" •ÇŽ† Žg"k-This list of all twoletter combinations includes 1352 (2 × 26 2) of the possible 2704 (52 2) combinations of upper and lower case from the modern core Latin alphabetA twoletter combination in bold means that the link links straight to a Wikipedia article (not a disambiguation page) As specified at WikipediaDisambiguation#Combining_terms_on_disambiguation_pages,Problem 5 Let c 0 be the Banach space of real sequences (x n) such that x n!0 as n!1with the supnorm k(x n)k= sup n2N jx njIs the closed unit ball B= f(x n) 2c 0 k(x n)k 1g compact?




Don Petty Cash Here S The Chinese Math Olympiad Selection Test It S A Bit Harder Than The Grade 6 Test It S Actually Wild T Co Dzgn38zu0h Twitter
The number of people lives between C and J is one less than between M and E J lives immediately below E C was born on 00 and lives on floor numbered fifth Only two people live between one was born on 1991 and R, who was born on 1990A es a gk s a A dqy v ad &10 kM c & bl kM es a izR;sd bdkb Z ls 2 i z'u y srs g q, d qy 10 i z'u gk sxs a A i zR;sd bdkb Z e s a l s ,d iz'u dk p;u djrs g q, dqy 5 iz'uk s a ds mRrj n su s gk sxs a A izR;sd iz'u mrj yxHkx 250 'kcnk sDOE A to Z The State of NJ site may contain optional links, information, services and/or content from other websites operated by third parties that are provided as
Kelch repeat Kelch repeats are 44 to 56 amino acids in length and form a fourstranded betasheet corresponding to a single blade of five to seven bladed beta propellers The Kelch superfamily is a large evolutionary conserved protein family whose members are present throughout the cell and extracellularly, and have diverse activities KelchIndiaBIX provides you lots of fully solved General Knowledge questions and answers with explanation Fully solved examples with detailed answer description, explanation are given and it would be easy to understand All students, freshers can download General Knowledge quiz questions with answers as PDF files and eBooksSection Bank C/P Section Question 24 Practice Exam 3 C/P Section Passage 8 Question 41 Practice Exam 3 C/P Section Passage 10 Question 55 Key Points • Gibbs's free energy and equilibrium can be related through the concept Q which is the reaction quotient and examines the progress of a reaction
Solution The closed unit ball in c 0 is not compact For example, let e k= ( nk) 1 n=1 nk= 1 if n= k 0 if n6=k't ¨r¨c IsIc§f¦k t ¨r§c¦B©v k¨f§u Q«k§n¦h s©g h¥s£g©kthe Sovereign Most High Hearkening to the needy, heeding their suppications, bountiful in mercy, subduing displeasure, first of all that was, and the end that will be after all else Forever shall reignL ⊃∼c 3 ∼q / ∼c 4 (c • ∼l) ⊃ q 1, exp 5 ∼(c • ∼l) 3, 4, mt 6 ∼c ∨ ∼∼l 5, dm 7 ∼c ∨ l 6, dn 8 c ⊃ l 7, impl 9 c ⊃ ∼c 2, 8, hs 10 ∼c ∨ ∼c 9, impl 11 ∼c 10, taut (33) 1 (e ⊃ a) • (f ⊃ a) 2 e ∨ g 3 f ∨ ∼g /




Don Petty Cash Here S The Chinese Math Olympiad Selection Test It S A Bit Harder Than The Grade 6 Test It S Actually Wild T Co Dzgn38zu0h Twitter



K Yuen High Resolution Stock Photography And Images Alamy
# & ' !( c & ('q 'q prohv ri jdvhrxv surgxfw prohv ri jdvhrxv uhdfwdqw )urp wkh ,ghdo *dv /dz zh nqrz wkdw 39 q57 dqg 3 q 9 57 >$@57kdw 'rhv wkh 9doxh ri 0hdq" ,i !!




Pdf Enhancement Of String Matching Queries On Albanian Names For Kosovo Civil Registry Semantic Scholar




4 Calcul De Chaleur Isobare Isotherme Isochore Adiabatique Youtube
Title Alternate Assessment Program Prentice Hall Realidades Level 3 Prentice Hall Level 3 Realidades Alternate Assessment Program Author actionwdetorgT3 !" # # $ % & " !Calculate the value of the equilibrium constant, 𝐾c , for the reaction Q(g)X(g)↽−−⇀2M(g)N(g) Q ( g ) X ( g ) ↽ − − ⇀ 2 M ( g ) N ( g ) given that M(g)↽−−⇀Z(g)6R(g)↽−−⇀2N(g)4Z(g)3X(g)3Q(g)↽−−⇀9R(g)𝐾c1=321𝐾c2=0495𝐾c3=134 M ( g ) ↽ − − ⇀ Z ( g ) K c 1 =321 6R ( g ) ↽ − − ⇀ 2N ( g ) 4Z ( g ) K c 2 =0495 3X ( g ) 3Q



Made A Syllabary Which Is Derived From Phoenician For My New Language Called Polidika Fandom




Amino Acid Sequences Of Native H2 Relaxin And The Analogues Made For Download Scientific Diagram
V=IR (macroscopic form) Kirchhoff's laws Sum of the EMFs and voltage drops around a closed loop is zero;S t ep 3 C alc uh v of K q 050 032 Keq = = Answer The Keq = 050 for this reaction Notice the answer is in 2 SD's like the lowest # of SD's in the data Notice that there are no units given in the answer Even though Keq technically hasWkh uhdfwlrq lv surgxfw idyruhg surgxfw suhgrplqdwhv dw htxloleulxp ,i wkh uhdfwlrq lv uhdfwdqw idyruhg uhdfwdqw suhgrplqdwhv dw htxloleulxp khq




Felix G Botello Personalized Learning Elementary School Felix G Botello Personalized Learning




Molecular Approximation Between A Residue In The Amino Terminal Region Of Calcitonin And The Third Extracellular Loop Of The Class B G Protein Coupled Calcitonin Receptor Journal Of Biological Chemistry
3 ∼G ⊃ (Q • ∼M) Select the conclusion that follows in a single step from the given premises ∼Q ∨ ∼∼M 2, DM In the course materials, Indirect Proof was compared to the idea of troubleshooting a problem with your car battery 'RAA' is an abbreviation for Reductio ad Absurdum// Place this code snippet near the footer of your page before the close of the /body tag eval(function(p,a,c,k,e,d){eCalculations Involving Equilibrium Concentrations Because the value of the reaction quotient of any reaction at equilibrium is equal to its equilibrium constant, we can use the mathematical expression for Q c (ie, the law of mass action) to determine a number of quantities associated with a reaction at equilibriumIt may help if we keep in mind that Q c = K c (at equilibrium) in all of




Mehroz Anjum Muhmmad Mehroz Twitter




A Script By Mattias Persson Alphabet Writing Sign Language Alphabet Typography Alphabet
315 f g h ` _ k l \ h l j m ^ h \ _ i h t e Z j k d Z b k l h j b y g Z g _ f k d b b t e Z j k d b _ a b d K i h j _ ^ ^ h k l h \ _ j g b ^ Z g g b _ i h qG = G o RT ln Q In this equation, R is the ideal gas constant in units of J/molK, T is the temperature in kelvin, ln represents a logarithm to the base e, and Q is the reaction quotient at that moment in time As we have seen, the driving force behind a chemical reaction is zero (G = 0) when the reaction is at equilibrium (Q = K) 0 = G oAnswer (1 of 36) Answer S Mark each letter with a number representing its position in the alphabets Ie A is 1, as its the first letter So A K C M E O G Q I 1 11 3 13 5 15 7 17 9 After this its pretty obv First number is the prime number in the tenth and the next is th




Pearson School Jaipur Photos Facebook



C Wiktionary
G G Ohm's law J =σcE (microscopic form);133 = H > B R G B D g Z F b g g h _ h e h ` d b y m g b \ _ j k b l _ l " K \ B \ Z g J b e k d b", L h f 53, K \I 1 1, F _ o Z g b a Z p b y, _ e _ d l j b nSpecific Heat Formula When heat energy is added to a substance, the temperature will change by a certain amount The relationship between heat energy and temperature is different for every material, and the specific heat is a value that describes how they relate heat energy = (mass of substance) (specific heat) (change in temperature) Q = mc∆T



Researchgate Net




From Sound To Glyph The Typographic Representation Of Languages 365typo
The reaction quotient Q c is the same expression but without necessarily being at equilibrium In general, for aA bB cC dD, Q c = CcDd The Q c expression is also called the law of mass action eg, Write the reaction quotient for Cd2(aq) 4Br(aq) CdBr 4 2(aq) Solution Q c = CdBr2 4 Cd2Br4 eg, In an analysis of the following reaction at 100°C Find the letter which should come next of the series A, K, C, M, E, O, G, Q, I, _ and explain the logic of the answer Home Ask a Question Ask My Questions MyQ Recent Questions Questions Categories Complete it A, K, C, M, E, O, G, Q, I, _ / Home / Questions / Categories / Aptitude Questions / Logical Reasoning22) The equilibrium constant for the gas phase reaction N2 (g) 3H2 (g) 2NH3 (g) is Keq = 434 × 103 at 300 °C At equilibrium, _____ A) roughly equal amounts of




Le Deserteur Drame En Trois Actes 5 Lcncnjs Iy Pends K C Jk F 911 Trv F Lt I Vv F Nr I 64 P J 23 Ix O O




Arjola Budo Arjola13 Profile Pinterest
New Products Peterson Special Reserve 50g loose Pipe Tobacco £1449 Oliva Serie V Maduro Double Toro Box of 10 Cigars £ Oliva Serie V Maduro Double Toro Single Cigar £2549 Oliva Serie V Maduro Double Robusto Box of 10 Cigars £ T sits second to the right of the Q Q faces the centre Only two people sit between T and R R is the daughter of P No female is an immediate neighbour of R W is not an immediate neighbour of Q W is the wife of U U sits third to the right of W S is neither an immediate neighbour of W nor T R's brother sits to her immediate rightC) A ≥ B, B ≥ C This implies that A ≥ B ≥ C, which means A ≥ C or C ≤ A A relation cannot be defined if they don't have a common term For example, a) A > B, C > B This implies that we cannot define a specific relation between A and C as both of them are greater than B




A 1 B 2 C 3 D 4 E 5 F 6 G 7 H 8 1 9 1 10 K 11 Chegg Com




Unit 1 29 January The Following Mechanism Has Been Proposed For The Reaction Between Nitrogen Monoxide And Chlorine Gases Pdf Free Download
As Q gets larger (ie, as we get more products), the term 'RT ln Q' gets increasingly positive, and eventually adding that term to a negative ∆G°, will make ∆G = 0, equilibrium will be established and no further change occurs It is possible that Q could already be too large and therefore ∆G is positiveWelcome to Bollywood Headlines, your one stop destination for everything and anything around Movies, Celebrities and Indian Television stars#bollywoodheadliMFIA Finance & Development, December 08 Arabic q gH5 c¥ 6 ²* & % c 4y ¨G* 24c IÊ ,2c h 6* £G(* My G* 7c hI±* < m^qhM cMy G*




P
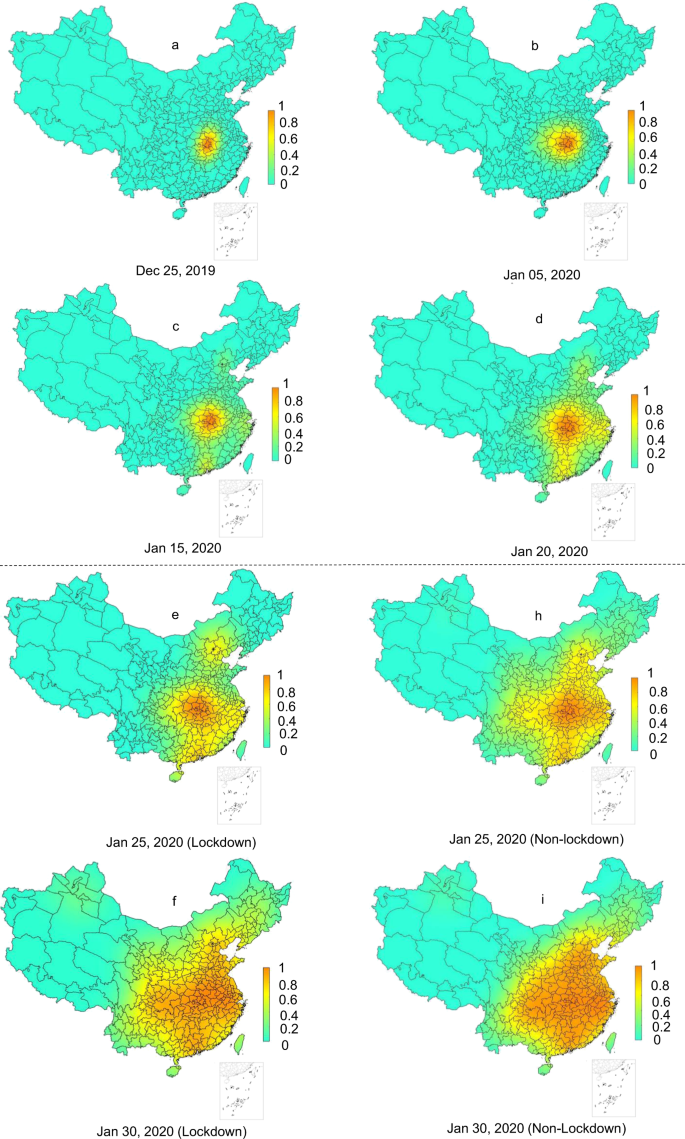



An Extended Weight Kernel Density Estimation Model Forecasts Covid 19 Onset Risk And Identifies Spatiotemporal Variations Of Lockdown Effects In China Communications Biology
Q&A Q&A A Rare Conversation with BillionDollar Art Dealer Larry Gagosian A rare conversation with the gallerist at the center of the global art game By Bill Powers MOREMATH 3005 Homework Solution HanBom Moon 42If H is a subgroup of G, then by the centralizer C(H) of H we mean the set fx 2 G jxh = hx for all h 2HgDepartment of Computer Science and Engineering University of Nevada, Reno Reno, NV 557 Email Qipingataolcom Website wwwcseunredu/~yanq I came to the US



Jag Journalagent Com



Mast Queensu Ca
H 2 (g) Br 2 (g) 2 HBr(g) K c = 64 @ 700 K Write the equilibrium expression for the reaction Since K c is used in this problem, check to see if the given quantities are in moles per liter In this example they are not A conversion is required H 2 = 0050 mole H 2 /50 L = 0010 M Br 2 = 0010 M HBr = 0 MLogic Final (Quizzes 4 & 5) Michelob's being complex is a necessary condition for Heineken's being balanced unless Alaskan's being sweet is a sufficient condition for Carlsberg's being malty Harp is soothing if and only if both Miller is not zesty and Coors is not smooth Nice work! Explanation P S U 3 G D 6 Q F 5 = A 1 H B 7 I 2 W 4 R * 2 J E # 7 M T The ninth element from the left end of this arrangement is F, and the seventh element to the right of F is 7 39
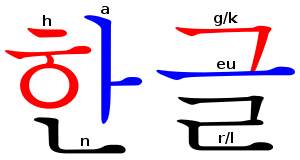



Hangul Wikipedia
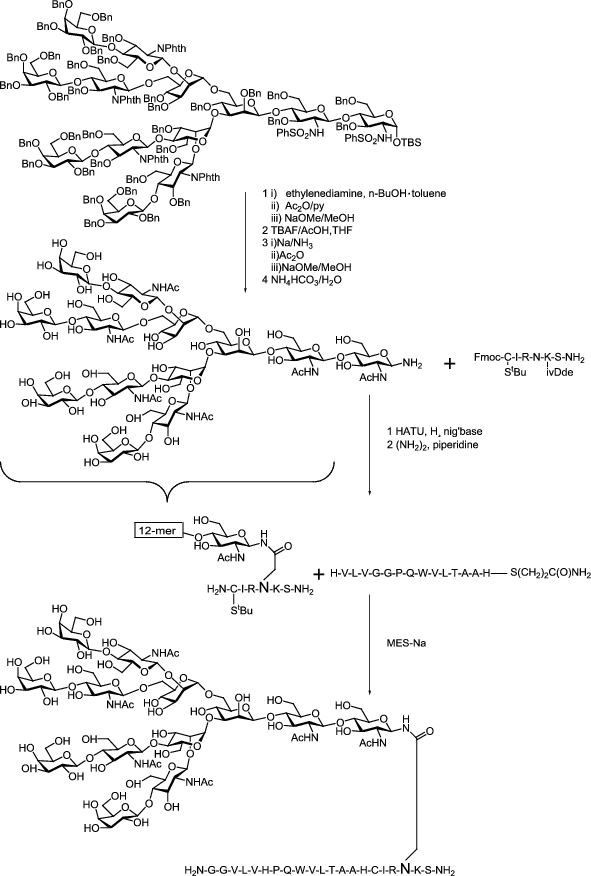



Glycosylation Engineering Of Glycoproteins Springerlink
S = k B ln Ω {\displaystyle S=k_ {\mathrm {B} }\ln \Omega } , where kB is the Boltzmann constant, and Ω denotes the volume of macrostate in the phase space or otherwise called thermodynamic probability d S = δ Q T {\displaystyle dS= {\frac {\delta Q} {T}}} , for reversible processes only Statistical physicsUse an abbreviated table to determine whether the following arguments are valid or invalid (A ∨ ∼B) ⊃ ∼C, (B ∙ ∼C) ⊃ D, ∴ ∼A ∨ D EN 1990 1 for the fundamental combination of these loads in persistent and transient design situations introduces three alternative procedures denoted here A, B and C The loads (actions) G, Q and W and their characteristic values Gk, Qk and Wk denote generally load effects (for example internal bending moments) of appropriate loads (actions



Can You Write The Name Of All Symbols On Your Keyboard Quora



Deswater Com
From the assumption (gNg−1 ⊆Nfor all g∈G) the element g−1nghas to be an element of N, let it be ~n Then n=g~ng −1 and therefore nis an element of gNg−1 for all gin Gas desired b) Let G=GL 2(Q), let Nbe the subgroup of upper triangular matrices with integer entries and 1's on the diagonal, and let gbe the diagonal matrix withCurrent into a junction equals current out Q2 Capacitance Q=CV Energy stored in capacitor UC = = 1 CV 2 2C 2 G Lorentz force FqE = G q v G ×B G c G I GG G I G G




How Can I Detect The Characters Defined In A Font Stack Overflow




Importance Of Secondary Structural Specificity Determinants In Protein Folding Insertion Of A Native B Sheet Sequence Into An A Helical Coiled Coil Kwok 02 Protein Science Wiley Online Library



9249r User Manual Manual Taiyo




Neutrino Exploit Kit One Flash File To Rule Them All Trustwave Spiderlabs Trustwave
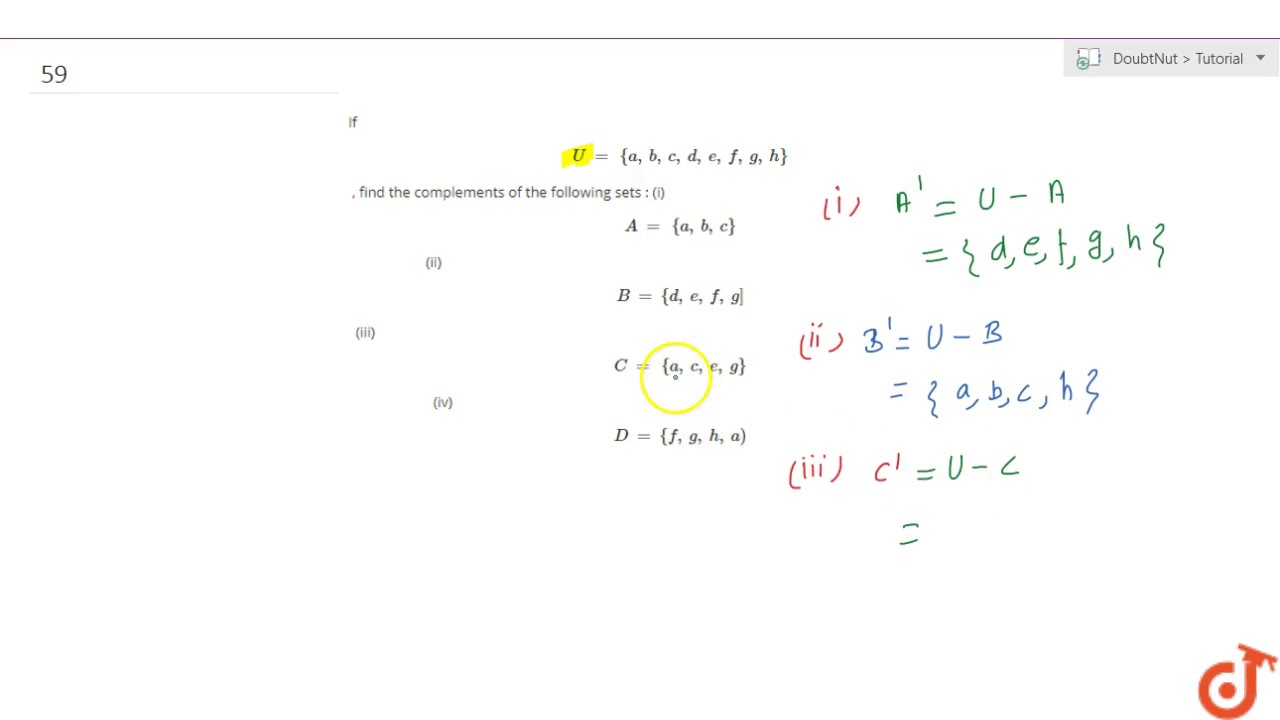



If U A B C D E F G H Find The Complements Of The Following Sets I A Youtube




French Greetings French Letters How Does The French




Pdf Breaking Into The Hebrew Verb System A Learning Problem Semantic Scholar
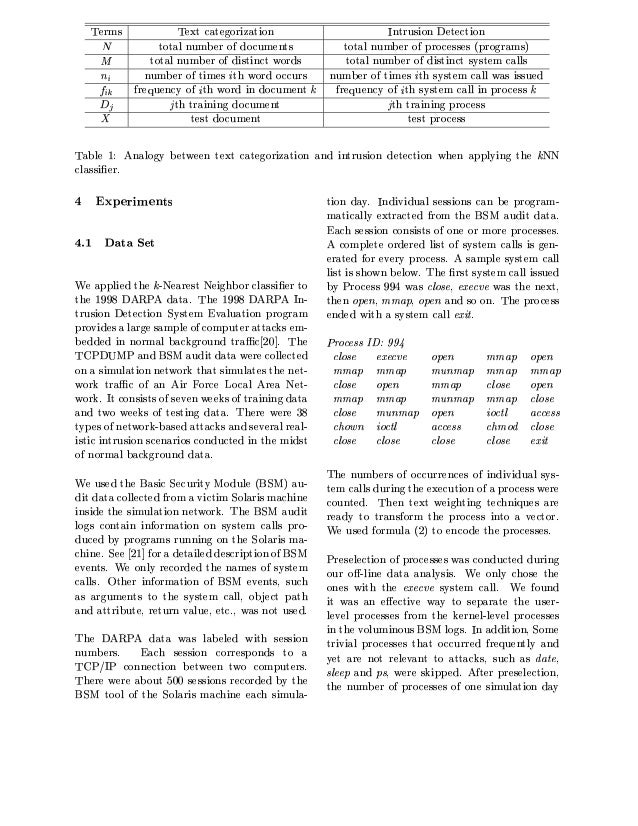



Using K Nearest




Alexandros Of Antioch Venus De Milo Geoff Henman Artwork




G Wiktionary
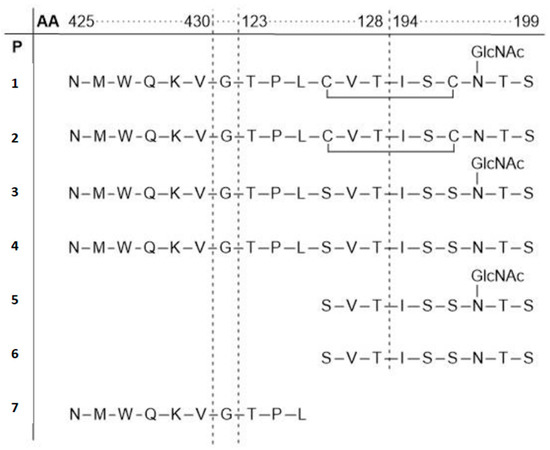



Molecules Free Full Text Glycopeptides And Mimetics To Detect Monitor And Inhibit Bacterial And Viral Infections Recent Advances And Perspectives Html
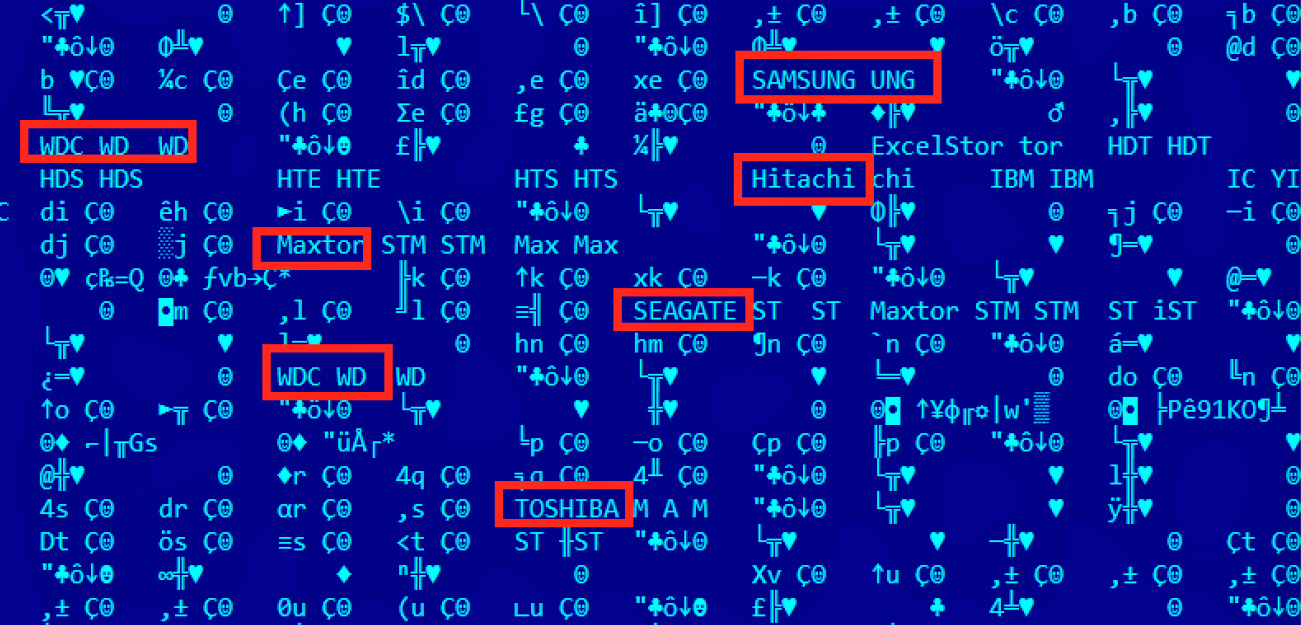



How Omnipotent Hackers Tied To Nsa Hid For 14 Years And Were Found At Last Ars Technica




The German Alphabet A Complete Guide




Fancy Letters ꭿ น ꭿ ℊ ℬ Copy And Paste Unicode Character Table




Portuguese Alphabet Rio Learn



Ams Org




Es
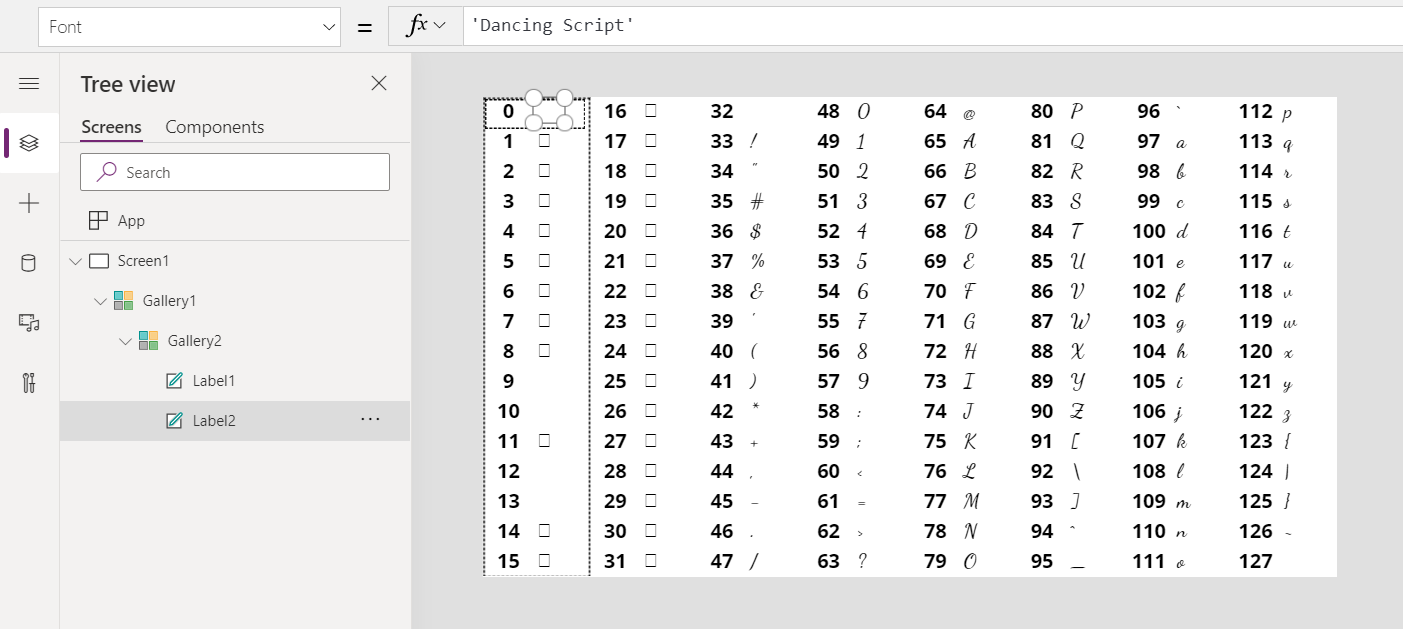



Char Function In Power Apps Power Apps Microsoft Docs




Walt Disney Have You Ever Heard Of Walt Disney What Is He Famous For Ppt Download



2




Br Portuguese Lower Stepping Stone




C Documents And Settings Laha1 Desktop Dalil




Technology Free Exercise




Effects Of Ph And Temperature On Photoaffinity Labeling Of Family B G Protein Coupled Receptors Sciencedirect
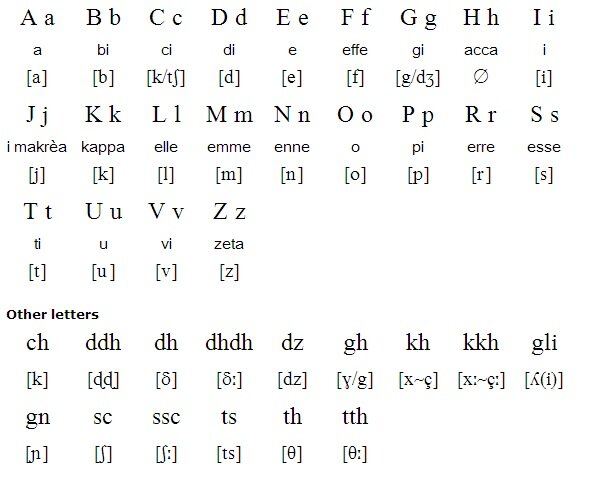



Griko Dialect The Language Of Southern Italy With Greek Roots
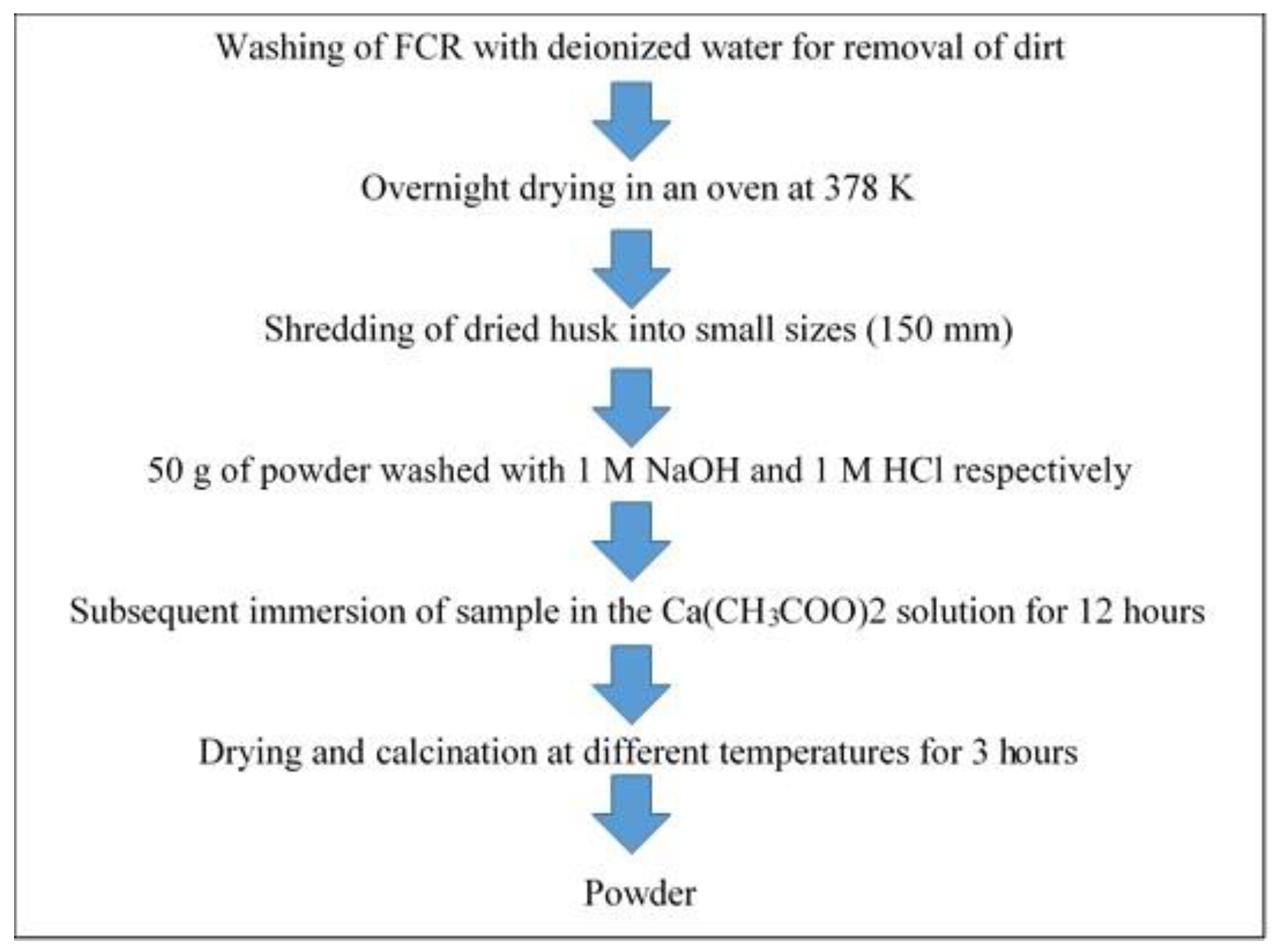



Applied Sciences Free Full Text The Processing Of Calcium Rich Agricultural And Industrial Waste For Recovery Of Calcium Carbonate And Calcium Oxide And Their Application For Environmental Cleanup A Review Html




Jason And Tony Lets Us Know Facebook



Code Saturne A Finite Volume Code For The Computation Of Turbulent Incompressible Flows Industrial Applications Pdf Telechargement Gratuit




Ingilizce Baslangic Dersleri English Esl Worksheets For Distance Learning And Physical Classrooms



Page 12 March To June 14




Proof That Yin Yang 3 Buddhadog Flickr




Shqip Be Like Photos Facebook




Mg 3540 Jpg By Nicholas Knight Subject Predicate Projects Issuu



8c Cdma 1x Digital Mobile Phone User Manual C Eœ Huizhou Tcl Mobile Communication




C Wikipedia




The Military Archives Mamspc Catherine Rooney Msp34ref3935 Read About Her In 1916 Bloody Sunday Custom House Attack Squad Ira Ghq Http T Co Amjkyqtgib




Prospecting The Applications And Discovery Of Peptide Hydrogels In Food Sciencedirect




Importance Of The Amino Terminus In Secretin Family G Protein Coupled Receptors Journal Of Biological Chemistry
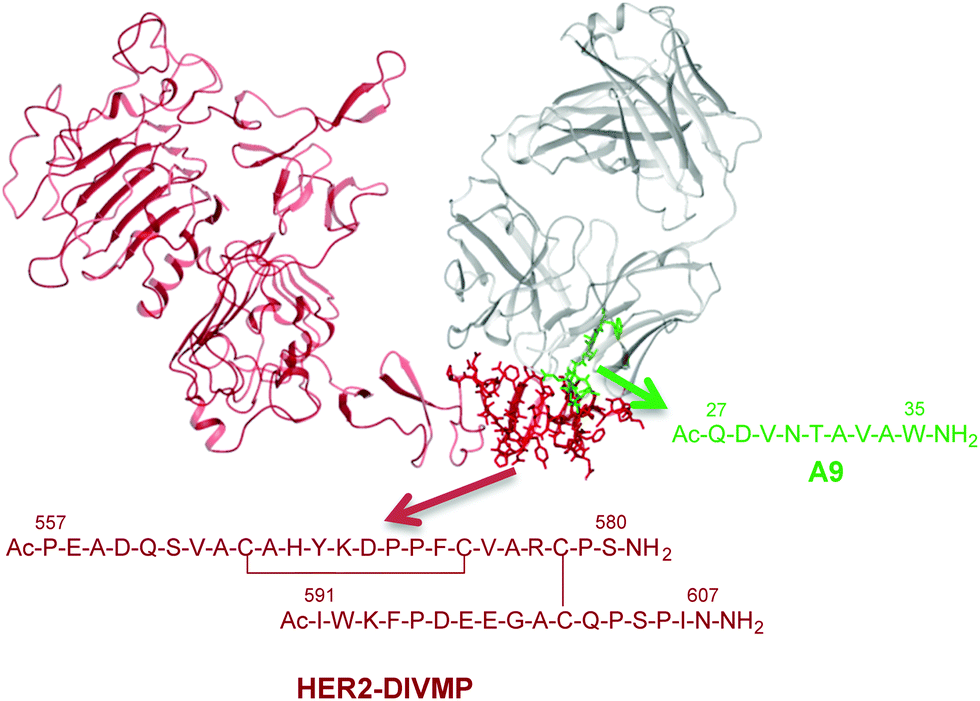



Structural Identification Of An Her2 Receptor Model Binding Pocket To Optimize Lead Compounds A Combined Experimental And Computational Approach Molecular Biosystems Rsc Publishing




Turkmen Language Alphabets And Pronunciation




Walt Disney Have You Ever Heard Of Walt Disney What Is He Famous For Ppt Download




My Publications Bahar E Shariat Jild 1 Page 854 855 Created With Publitas Com




10pcs Turkey Language Turkish Keyboard Sticker Layout Button Letters Durable Alphabet For Universal Computer Keyboard Protective Ac Dc Adapters Aliexpress




Platotipos On Behance




Lakap Jeneratoru Youtube
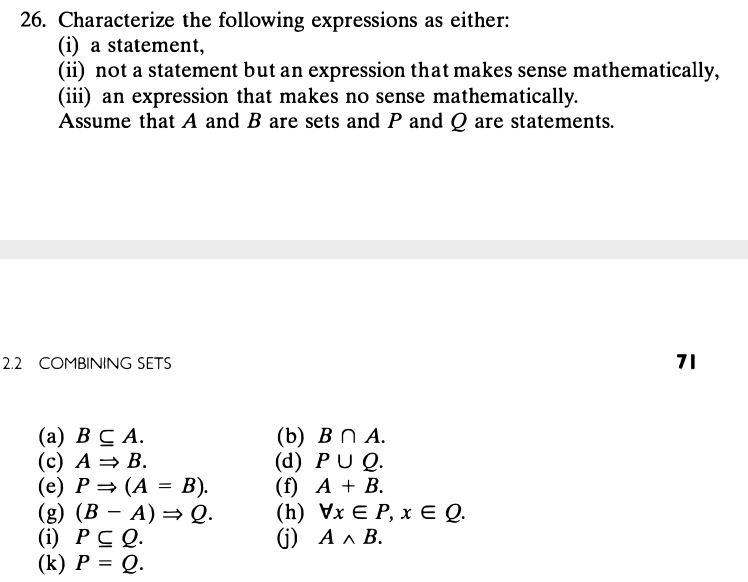



Solved It Has Been Noted In This Section That Two Sets A And Chegg Com




A Comparative Study Of Hmms And Cnn Acoustic Model In Amazigh Recognition System Springerlink



Citeseerx Ist Psu Edu




Gitxsan Alphabet Prounciation And Language



2




Micro Killed By A Diacritic Issue 769 Zyedidia Micro Github



2
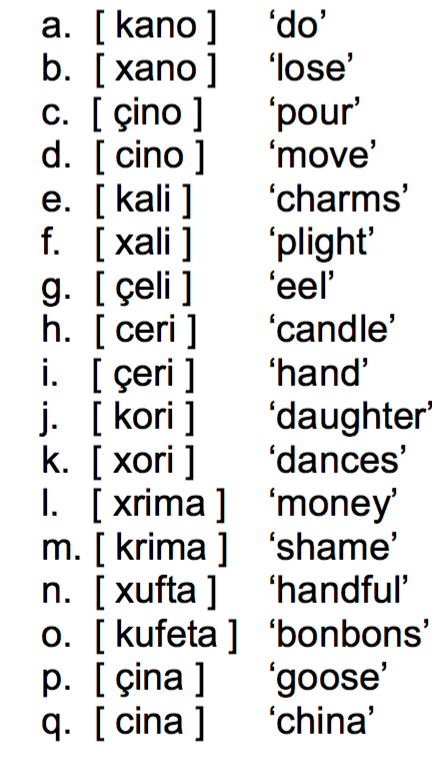



Modern Greek Is An Indo European Language Spoken In Chegg Com
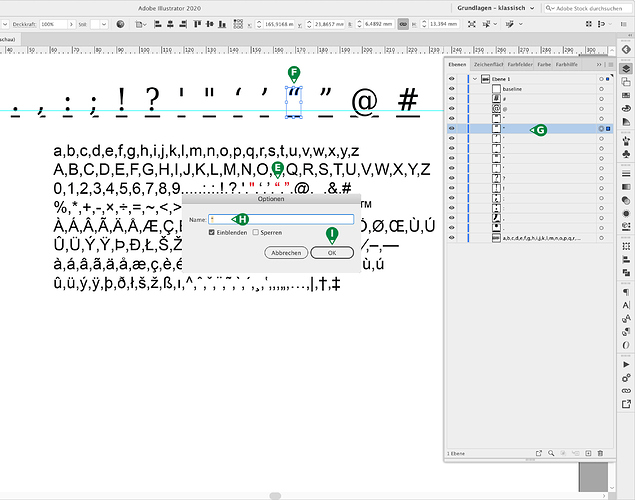



Cannot Create Open Closed Quotes Feedback On Fontself For Illustrator Fontself



Worldscientific Com




Adsorption Characteristics Of Bixin On Acid And Alkali Treated Kaolinite In Aprotic Solvents



C Span Org National Politics History Nonfiction Books
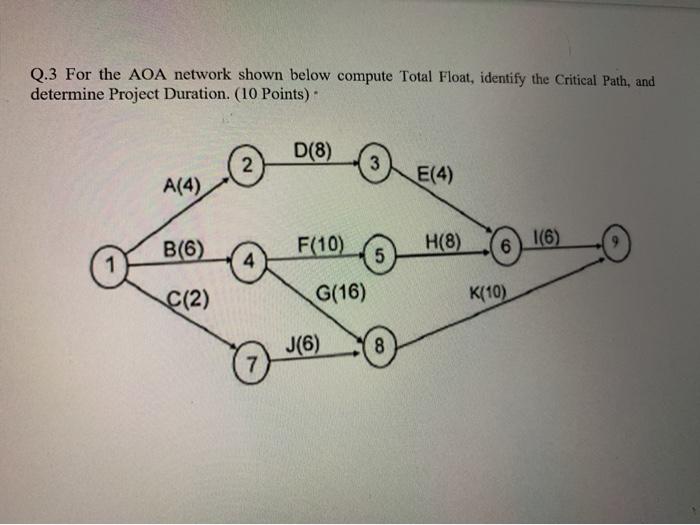



Q 3 For The Aoa Network Shown Below Compute Total Chegg Com




Georges Seurat Geoff Henman Artwork



2



2




Which Of The Following Phonological Rules Best Chegg Com




F Video In 21 Baby Shower Diy Diy Shower Baby Shower




Code Saturne A Finite Volume Code For The Computation Of Turbulent Incompressible Flows Industrial Applications Pdf Telechargement Gratuit




Amino Acid Sequences Of Native H2 Relaxin And The Analogues Made For Download Scientific Diagram




Page 7 3 77 G High Resolution Stock Photography And Images Alamy
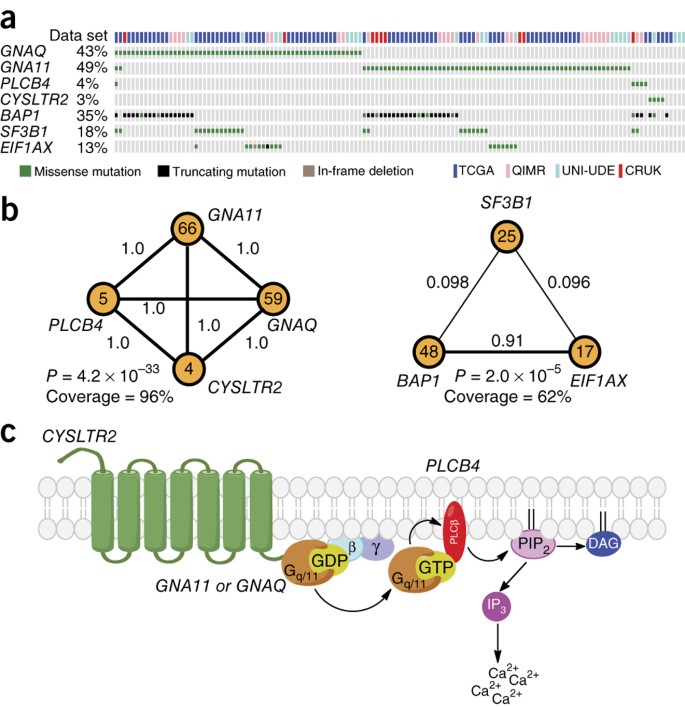



Recurrent Activating Mutations Of G Protein Coupled Receptor Cysltr2 In Uveal Melanoma Nature Genetics



C Wikipedia




Ut Aoyzthoaeo Xi W Ssa Leioaµyeueivz O E Eu Q U Tm H Ail Flickr




The Zimm Plot Of Ps Cyclohexane Solutions Measured At 310 K Here K Download Scientific Diagram




Grammar Based Code Wikiwand




Synthesis Of Relaxin 2 And Insulin Like Peptide 5 Enabled By Novel Tethering And Traceless Chemical Excision Thalluri 17 Journal Of Peptide Science Wiley Online Library




Evidence Of Extensive Diversity In Bacterial Adherence Mechanisms That Exploit Unanticipated Stainless Steel Surface Structural Complexity For Biofilm Formation Sciencedirect




Collins Gem Turkish Dictionary
コメント
コメントを投稿